
| Home | About Us | Contribute | Bookstore | Advertising | Subscribe for Free NOW! |
| News Archive | Features | Events | Recruitment | Directory |
| FREE subscription |
| Subscribe for free to receive each issue of Semiconductor Today magazine and weekly news brief. |
News
8 December 2008
Probing hydrogen’s impact on zinc oxide
Zinc oxide (ZnO) would be a perfect low-cost solution to light emission but for one thing - it is extremely difficult to find suitable doping processes for creating p-type material (i.e. with holes, rather than electrons, as the majority carrier). Light emission, for example, depends on the ability to bring together electrons (from n-type material) and holes (from p-type material) so that transitions across ZnO’s 3.4eV energy bandgap can take place, producing ultraviolet light that can be used with phosphors to produce white light.
ZnO’s wide bandgap is comparable with the GaN semiconductor system widely used for short-wavelength/white light and blue laser emission devices. Some UV laser diodes have been produced in ZnO, along with novel electronics devices, some of which are based on the material’s piezoelectric properties. ZnO is also being developed as a transparent conductive oxide to replace expensive alternatives such as indium tin oxide (ITO). While zinc is the 23 rd most abundant element on earth, gallium only occurs in trace amounts of the order of tens of parts per million (e.g. ~50ppm in bauxite and zinc ores) and is extracted as a byproduct of aluminum and zinc production.
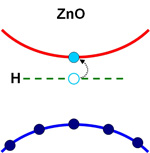 The main problem is that, when it is not intentionally doped, ZnO tends to be strongly n-type, creating a large compensation that needs to be overcome before p-type materials can be reached. Finding out the nature of the n-type unintentional doping could lead to more effective methods of creating p-ZnO, opening up new opportunities for lower-cost ZnO-based electronics and light emission.
The main problem is that, when it is not intentionally doped, ZnO tends to be strongly n-type, creating a large compensation that needs to be overcome before p-type materials can be reached. Finding out the nature of the n-type unintentional doping could lead to more effective methods of creating p-ZnO, opening up new opportunities for lower-cost ZnO-based electronics and light emission.
Picture: Hydrogen atoms have been shown to always result in n-doped ZnO.
It is believed that hydrogen contributes to the n-type character of ZnO crystals. Hydrogen is almost impossible to avoid in semiconductor processing without special measures being taken. Its incorporation in bulk ZnO increases the material’s conductivity – indeed, such an effect has been used to construct hydrogen detectors.
Now, a German research team consisting of scientists at Ruhr-University Bochum and University Erlangen-Nuremberg have performed high-resolution electron energy loss spectroscopy (HREELS) measurements on hydrogen-treated ZnO substrates to disentangle the nature of the doping effect of hydrogen in zinc oxide [Qiu et al, Phys. Rev. Lett., vol.101, p.236401, 2008].
Compared with previous research, the researchers found a lower ionization energy (~25meV) for hydrogen as a donor of electrons to the conduction band. This compares with the energy value of about 26meV that is equivalent to the temperature of 300K, suggesting a high ionization rate at room temperature.
HREELS involves scattering electrons from materials and determining the properties of the energy loss from inelastic scattering. Since the mean free path of the incident electrons corresponds to a few monolayers of material, the technique reveals the surface properties.
The ZnO(000-1) substrate was prepared with oxygen as the termination by using suitable sputtering and annealing processes. The O-ZnO surface reacts with water vapor, forming a stable HO-terminated surface. The HO-surface adds a new peak to the energy loss spectrum at 449meV, associated with a stretching vibration of the HO formation.
The quasi-elastic peak around zero energy loss is related to an interaction between the incident electrons and plasmon states in the material under study. The plasmon energy is in turn related to the carrier density. For the HO-terminated wafer the full-width at half maximum (FWHM) of the quasi-elastic peak of 6meV corresponds to a reasonably low carrier density of 9x1013/cm3.
After exposure to atomic hydrogen (from H2 dissociated by a tungsten filament), the peak broadens to 24meV at 300K, corresponding to a carrier density of 1x1017/cm3. The hydrogen atoms are believed to enter interstitial positions between the zinc and oxygen lattice sites. One reason for hydroxylating the surface is to avoid an H-induced surface state that was posited by another group as being the explanation for the quasi-elastic peak broadening.
When the substrate is annealed after exposure to hydrogen the broadening decreases steadily with annealing temperature, indicating a reduction in the carrier density. These results from annealing are interpreted as showing desorption of the hydrogen from the substrate.
The temperature dependence of the quasi-elastic peak below room temperature (300K) was used to determine the ionization energy of the H atoms for producing electrons in the conduction band. This was found to be 25+/-5meV. This compares with 35+/-5meV, as determined by a group using electron paramagnetic resonance (EPR) spectroscopy. These values differ significantly with the traditional understanding dating back to 1957 that the ionization energy is about 51meV.
With a higher binding energy, the ionization rates at room temperature would be significantly lower. It is speculated by the German researchers that previous high values for the ionization energy might be due to contributions from defects, such as oxygen vacancies.
The researchers plan to use the technique with other forms of ZnO doping, particularly with metals. Some first tests have been made using copper, for example. There are also plans to use hydrogen-free substrates to produce p-type behavior with suitable dopants.
Professor Christof Wöll of Ruhr-University Bochum comments: “For the fundamental studies we are doing right now on 'perfect' model systems, nitrogen is best for p-doping because we can insert it in a very controlled fashion. Later we will test other elements, because for technical applications nitrogen is (probably) not well suited.”
See article: Charting routes to zinc oxide applications
![]() Search: ZnO Laser diodes GaN ITO
Search: ZnO Laser diodes GaN ITO
Visit: http://link.aps.org
The author Mike Cooke is a freelance technology journalist who has worked in the semiconductor and advanced technology sectors since 1997.