- News
23 July 2015
ORNL makes scalable arrays of heterojunctions within nanometer-thick 2D crystalline monolayer
The US Department of Energy's Oak Ridge National Laboratory (ORNL) has for the first time, it is claimed, combined a novel synthesis process with commercial electron-beam lithography techniques to produce arrays of semiconductor junctions in arbitrary patterns within a single, nanometer-thick semiconductor crystal (Mahjouri-Samani et al, 'Patterned Arrays of Lateral Heterojunctions within Monolayer Two-Dimensional Semiconductors', Nature Communications 6, 7749).
The process relies on transforming patterned regions of one existing single-layer crystal into another. The researchers first grew single, nanometer-thick layers of molybdenum diselenide crystals on substrates and then deposited protective patterns of silicon oxide using standard lithography techniques. They then bombarded the exposed regions of the crystals with a laser-generated beam of sulfur atoms. These replaced the selenium atoms in the crystals to form molybdenum disulfide, which has a nearly identical crystal structure. The two semiconductor crystals formed sharp heterojunctions.
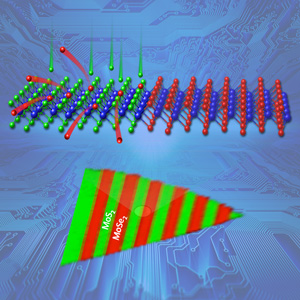 Complex, scalable arrays of heterojunctions formed within a 2D crystalline monolayer of molybdenum deselenide by converting lithographically exposed regions to molybdenum disulfide using pulsed laser deposition of sulfur atoms. Sulfur atoms (green) replaced selenium atoms (red) in lithographically exposed regions (top), as shown by Raman spectroscopic mapping (bottom). Image credit: ORNL.
Complex, scalable arrays of heterojunctions formed within a 2D crystalline monolayer of molybdenum deselenide by converting lithographically exposed regions to molybdenum disulfide using pulsed laser deposition of sulfur atoms. Sulfur atoms (green) replaced selenium atoms (red) in lithographically exposed regions (top), as shown by Raman spectroscopic mapping (bottom). Image credit: ORNL.
"We can literally make any kind of pattern that we want," says Masoud Mahjouri-Samani, who co-led the study with David Geohegan (head of ORNL's Nanomaterials Synthesis and Functional Assembly Group at the Center for Nanophase Materials Sciences, and the principal investigator of a Department of Energy basic science project focusing on the growth mechanisms and controlled synthesis of nanomaterials). Millions of two-dimensional (2D) building blocks with numerous patterns may be made concurrently, Mahjouri-Samani adds. In the future, it might be possible to produce different patterns on the top and bottom of a sheet. Further complexity could be introduced by layering sheets with different patterns.
"The development of a scalable, easily implemented process to lithographically pattern and easily form lateral semiconducting heterojunctions within two-dimensional crystals fulfills a critical need for 'building blocks' to enable next-generation ultrathin devices for applications ranging from flexible consumer electronics to solar energy," says Geohegan.
Tuning the bandgap
"We chose pulsed laser deposition of sulfur because of the digital control it gives you over the flux of the material that comes to the surface," says Mahjouri-Samani. "You can basically make any kind of intermediate alloy. You can just replace, say, 20% of the selenium with sulfur, or 30%, or 50%," he adds. "Pulsed laser deposition also lets the kinetic energy of the sulfur atoms be tuned, allowing you to explore a wider range of processing conditions," notes Geohegan.
By controlling the ratio of sulfur to selenium within the crystal, the bandgap of the semiconductors can be tuned, determining the electronic and optical properties. To make optoelectronic devices such as electroluminescent displays, microchip fabricators integrate semiconductors with different bandgaps. For example, since molybdenum disulfide's bandgap is greater than molybdenum diselenide's, applying voltage to a crystal containing both causes electrons and holes (the positive charges created when electrons vacate) to move from molybdenum disulfide into molybdenum diselenide and recombine to emit light at the bandgap of molybdenum diselenide. For that reason, engineering the bandgaps of monolayer systems can allow the generation of light with many different colors, as well as enable other applications such as transistors and sensors, Mahjouri-Samani says.
Next, the researchers will see if their pulsed laser vaporization and conversion method will work with atoms other than sulfur and selenium. "We're trying to make more complex systems in a 2D plane — integrate more ingredients, put in different building blocks — because, at the end of the day, a complete working device needs different semiconductors and metals and insulators," says Mahjouri-Samani.
To understand the process of converting 1nm-thick crystal into another, the researchers used powerful electron microscopy capabilities available at ORNL (notably atomic-resolution Z-contrast scanning transmission electron microscopy, which was developed at the lab and is now available to scientists worldwide using the Center for Nanophase Materials Sciences). Electron microscopists Andrew Lupini and visiting scientist Leonardo Basile imaged hexagonal networks of individual columns of atoms in the nanometer-thick molybdenum diselenide and molybdenum disulfide crystals. "We could directly distinguish between sulfur and selenium atoms by their intensities in the image," Lupini says. "These images and electron energy loss spectroscopy allowed the team to characterize the semiconductor heterojunction with atomic precision."
Metal dichalcogenide heterostructure Molybdenum disulfide
www.nature.com/ncomms/2015/150722/ncomms8749/full/ncomms8749.html


