- News
27 December 2017
Indium metal-catalysis of gallium oxide plasma molecular beam epitaxy
Paul-Drude-Institut für Festkörperelektronik in Germany has developed epitaxy techniques for gallium oxide growth that could lead to sesquioxide heterostructures where the metal involved is aluminium (Al2O3), gallium (Ga2O3) or indium (In2O3) [Patrick Vogt et al, Phys. Rev. Lett., vol119, p196001, 2017]. In particlular, the team found that the presence of indium during plasma-assisted molecular beam epitaxy (PAMBE) increased the growth rate of Ga2O3, even when there is no growth without indium.
Gallium oxide has a wide bandgap (~5eV) and the researchers hope that their work will be a path for bandgap engineering and heterostructural growth of transparent semiconducting oxides. They see analogies with the III-V world where heterostructures of semiconductor alloys have created a vast range of optoelectronic and telecom devices. For the sesquioxides, the team suggests applications in deep-ultraviolet detection, transparent transistors, and high-power electronics.
One problem is that the sesquioxides tend to crystallize in incompatible structures: corundum (rhombohedral, R-3c) for α-Al2O3, gallia (monoclinic, C2/m) for β-Ga2O3, and bixbyite (body centered cubic, Ia-3) for In2O3. Also, the complexity of the oxide reactions leading to sesquioxides restricts growth to lower temperatures, reducing crystal quality.
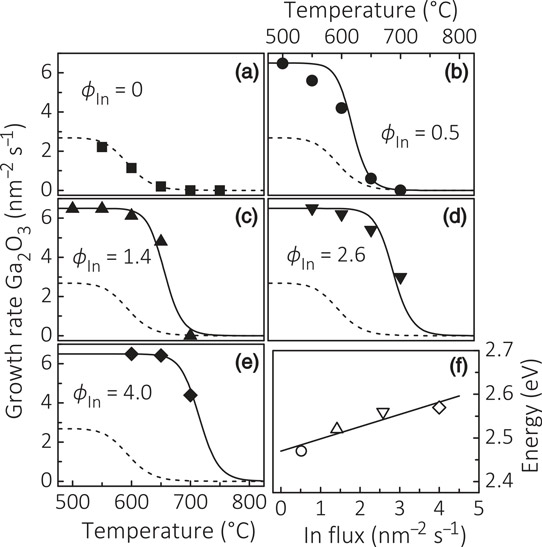
Figure 1: (a)–(e) Temperature dependence of Ga2O3 growth rate with varying indium flux (φIn in units of nm-2s-1). The gallium flux was 6.5/nm2-s. (f) Activation energy of indium desorption as function of φIn.
The researchers used PAMBE on the (0001) surface of α-aluminium oxide (sapphire) substrates. The growth started with 20nm β-Ga2O3 nucleation. In further growth, the team found that the presence of indium catalyzes the growth of Ga2O3 in 700°C conditions where no growth occurs in the absence of indium (Figure 1). At the same time, the indium is not incorporated into the growing Ga2O3 crystal structure. “This effect should not be confused with that of a surfactant, which either inhibits or induces a morphological phase transition but does not affect the growth rate of the material,” the researchers warn.
The team explains the effect in terms of two steps: first, the formation of In2O3 is kinetically favored over Ga2O3 by a factor of 2.8; second, however, In2O3 is unstable in the presence of gallium, energy factors favor indium replacement by gallium.
X-ray analysis suggested that the Ga2O3 grown with indium catalysis does not correspond to the underlying nucleation layer with the reflections shifted to larger angles. The team reports; “The angular position of these reflections perfectly agrees with those of the 0002 and 0004 reflections of the metastable ε-Ga2O3 phase, which crystallizes in a hexagonal structure (P63mc).”
Further x-ray and electron diffraction analysis confirmed the ε-Ga2O3 attribution, according to the researchers. “Clearly, the profiles reveal a well-oriented epitaxial film, which is particularly remarkable since films in the ε phase could not yet be obtained by PAMBE,” they add.
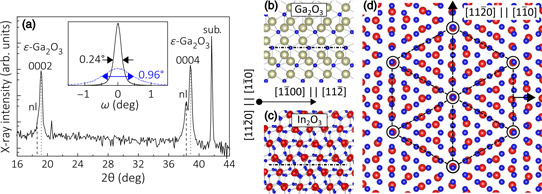
Figure 2: (a) Longitudinal x-ray diffraction scan of In2O3-catalyzed Ga2O3 700°C film with 5.4/nm2-s indium flux. Reflections labeled ‘sub.’ and ‘nl’ stem from substrate and nucleation layer, respectively. Inset: transverse scans across ε-Ga2O3 (0004) (solid black line) and (10-14) (dashed-dotted blue line) reflections with full-width at half maxima. (b) (c) Side-view schematics of ε-Ga2O3 (0001) and In2O3 (111) planes, respectively. Dashed-dotted lines indicate bulk-terminated surfaces. Ga, In and O atoms are depicted in gold, red and blue, respectively. (d) Top view of ε-Ga2O3 (0001) and In2O3 (111). Dashed lines illustrate 5∶4 coincidence lattice of O-terminated ε-Ga2O3 (0001) relative to indium-terminated In2O3 (111).
The researchers see the ε-Ga2O3 (0001) structure as being better suited to heterostructures involving In2O3 (111) since the planes match in terms of symmetry, atomic spacing, and surface chemistry. The researchers comment: “the two lattices are in almost perfect registry when forming a 5∶4 coincidence lattice with a residual mismatch of 1.3%.”
The researchers suggest that the metal-catalysis could apply to systems with similar kinetic and thermodynamic properties: “For example, we recently showed that the oxidation efficiency of Sn [tin] (ηSn) is even larger than that of In, which, in turn, is larger than that of Ga, i.e., ηSn > ηIn > ηGa. Thus, we expect catalytic effects for a wide range of ternary oxide alloys, but also for various other multi-component oxides fabricated by MBE.”
III-sesquioxide heterostructures AlO GaO InO PAMBE
https://doi.org/10.1103/PhysRevLett.119.196001
The author Mike Cooke is a freelance technology journalist who has worked in the semiconductor and advanced technology sectors since 1997.


