- News
21 June 2017
Finding the root of nanopipes in aluminium nitride on sapphire
Researchers in the USA and Japan suggest that precursor residues on reactor quartz-ware are responsible for large variations in the quality of aluminium nitride (AlN) films grown on sapphire by metal-organic vapor phase epitaxy (MOVPE) [D. D. Koleske et al, Appl. Phys. Lett., vol110, p232102, 2017].
The team from Sandia National Laboratories and JR Creighton Consulting LLC in the USA and Taiyo Nippon Sanso Corp in Japan was particularly keen to reduce the formation of nanopipes or open-core screw dislocations. Such nanopipes form current leakage paths that can kill light-emission performance in gallium nitride and aluminium gallium nitride devices. Such devices often use material grown on AlN nucleation layers.
The AlN was grown through MOVPE on sapphire using trimethyl-aluminium (TMAl) and ammonia (NH3) precursors in Taiyo Nippon Sanso high-temperature, high-pressure SR4000 system. The growth began with nitridation and 100nm AlN nucleation at 920°C, followed by 2.7μm AlN at 1320°C. The growth time was 50 minutes.
The low-temperature AlN nucleation layer was N-polar, while that of the high-temperature AlN was Al-polar. The researchers comment: “While this polarity inversion is not entirely understood, N- to Al-polarity inversion has been reported after annealing to 1650–1700°C in N2-CO gas mixtures. Also, recent work by Mohn et al has shown that thin oxynitride phases of AlxOyNz might provoke polarity inversion especially for AlN growth on oxide surfaces.”
The researchers found that exposing the quartz-ware components of the reactor to room air or water (H2O) vapor in a nitrogen-purged glovebox reduced etch pit densities (EPDs) to less than 100/cm2. Further, the full-width at half-maximum (FWHM) of the (0002) x-ray diffraction (XRD) peak was 200 arc-seconds. These values suggest that the density of ‘nanopipe’ open-core screw dislocations was of the same order as for GaN growth. Without treating of the quartz-ware, the EPD increased to 1.6x107/cm2 and the (0002) FWHM was 398arcsec. The (0002) reflection is affected by dislocations with a screw component.
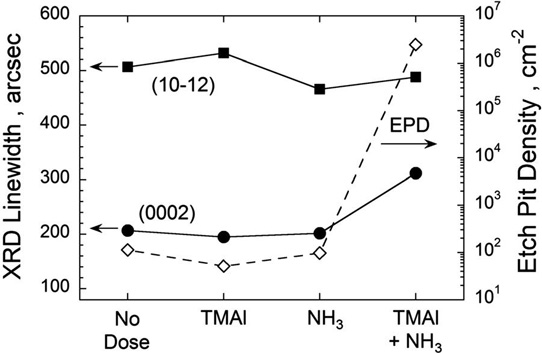
Figure 1: Plot of (0002) and (10–12) XRD linewidths (solid circles and squares, left axis) and EPD (open diamonds, right axis) as function of four different bake conditions. Quartz-ware was dosed with H2O for 30 minutes before baking to pre-condition quartz surfaces. After H2O exposure, quartz-ware was baked in hydrogen (H2) at 1300°C with 30-second dose of TMAl, NH3, or TMAl + NH3 midway during bake. For comparison, no-dosing condition is also shown.
The team comments: “Even small amounts of partly reacted TMAl and NH3 coatings on reactor components can strongly impact the AlN nucleation during the subsequent growth run as shown in [Figure 1]. Our work suggests that these unreacted TMAl + NH3 coatings, which should be present on the walls of other MOVPE systems, are significant factors in limiting reproducible growth of AlN on sapphire.”
The removable quartz-ware components were upper and lower quartz channels, a silicon carbide (SiC) coated susceptor, and a SiC susceptor cover. The researchers did not remove a quartz gas injector or exhaust funnel, since these were fixed in the reactor.
Scanning electron microscope (SEM) analysis showed that at least some of the open-core dislocations reached down to the AlN/sapphire interface. Potassium hydroxide (KOH) etching at 70°C was used to decorate/expand the open cores to make them more visible to optical Nomarski microscopic analysis (Figure 2).
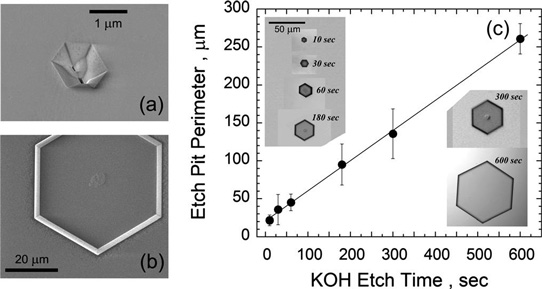
Figure 2: (a) SEM image of open core pit after AlN growth. (b) SEM image of KOH etched pit in AlN, showing hexagonal shape and possible unetched material in center. (c) Images of KOH etched pits for increasing etch times and plot of etch pit perimeter as function of KOH etch time.
The researchers comment: “We speculate that H2O reacts with TMAl and/or NH3 chemical moieties adsorbed on the reactor surfaces, rendering them inert for the next growth run.”
Deliberately dosing the quartz-ware with TMAl or NH3 separately did not increase the (0002) peak or EPDs. However, a combined dose gave AlN layers with 300arcsec FWHM and 1x106/cm2 EPDs. “These results suggest that the combined reaction of TMAl and NH3 results in a reactor coating that negatively impacts the subsequent AlN growth,” the team writes.
http://dx.doi.org/10.1063/1.4984900
The author Mike Cooke is a freelance technology journalist who has worked in the semiconductor and advanced technology sectors since 1997.


